How Does The Size Of The Uterus Change From Prepuberty To Menopause?
Continuing Didactics Activeness
Pelvic ultrasound is the imaging modality of choice to evaluate the uterus, cervix, ovaries, and adnexa. In the postmenopausal patient, it is performed for a variety of reasons, but most often for the evaluation of postmenopausal haemorrhage or an adnexal mass. This activity reviews pelvic ultrasound in the postmenopausal patient and highlights the interprofessional team's role in performing and evaluating these imaging studies.
Objectives:
- Describe the normal findings seen on ultrasound in postmenopausal women.
- Outline the workup of a patient with postmenopausal bleeding and the function of ultrasound in that evaluation.
- Review the ideal technique to measure endometrial thickness while performing pelvic ultrasound.
- Review how ultrasound tin be used to characterize adnexal masses in postmenopausal patients.
Introduction
Ultrasound is the outset imaging modality of choice for the female pelvis. It allows for real-time, fast, and portable imaging of the uterus, cervix, ovaries, and adnexa without the utilize of ionizing radiation. Here, we aim to summarize the use of pelvic ultrasonography in the postmenopausal population specifically.
Anatomy and Physiology
Changes After Menopause
In general, the same principles apply when imaging the postmenopausal pelvis in terms of beefcake.[ane] It is important to be aware of ultrasound changes after menopause due to normal hormonal changes.
Uterus
Excluding pathologies such as fibroids or adenomyosis, the postmenopausal uterus tends to exist smaller in overall size (as measured past uterine length, uterine height, and uterine width) compared to the reproductive historic period uterus. The uterus can continue to subtract in size throughout menopause.[2] Additionally, there is a reduction in the corpus-cervix ratio in postmenopausal women such that it returns to a configuration more similar to that seen in the pediatric or prepubescent uterus.[3] The normal postmenopausal endometrium should be sparse and appear atrophic.
Ovaries
Postmenopausal ovaries will also appear smaller and more homogenous, with occasional small hypoechoic follicles on ultrasound compared to those institute in reproductive-historic period women. Because of this, it tin be common non to visualize the ovaries on ultrasound in a postmenopausal woman, and this should not lead to business. Similarly to the uterus, the ovaries tin continue to decrease in volume through menopause.[2]
Indications
Some of the common indications for a pelvic ultrasound in postmenopausal women include postmenopausal haemorrhage, pelvic pain, history of ovarian cysts, abdominal bloating, increasing abdominal girth, or pelvic force per unit area.
Postmenopausal Bleeding
Postmenopausal bleeding is a mutual indication for ultrasound evaluation of the postmenopausal pelvis. Even though the most mutual cause of postmenopausal bleeding is atrophy of the vagina and/or endometrium due to decreased estrogen, it is the presenting symptom for nearly xc% of women with endometrial cancer.[4] Thus, it is of import to rapidly and accurately diagnose or exclude neoplasia in patients presenting with postmenopausal bleeding.
Transvaginal ultrasound measuring endometrial thickness is a reasonable first non-invasive footstep in evaluating a patient with postmenopausal haemorrhage. Through multicenter trials and retrospective meta-analyses, an endometrial thickness of 4mm has been well established equally the threshold above which endometrial sampling should be considered. With an endometrial thickness of 4mm or less, the negative predictive value for endometrial cancer is greater than 99%.[v] Smith-Bindman et al. conducted a meta-analysis of 35 studies including v,892 women to endorse this cut-off value. At varying endometrial thicknesses from 3mm to 10mm, the mean weighted pooled estimates for sensitivity and specificity were calculated. They were able to find that using a 5mm cutoff to ascertain abnormally thick endometrium was 96% sensitive for detecting endometrial cancer and 92% sensitive for detecting any endometrial disease (including polyps and hyperplasia). Other studies, such as Timmermans et al., found that a 3mm cutoff was ideal, having a sensitivity of 97.ix% for detecting endometrial cancer.[half-dozen] Yet, both these studies and others establish that endometrial thickness is not very specific for detecting endometrial cancer: at 5mm, Smith-Bindman et al. calculated 61% specificity, and at 3mm, Timmermans calculated but a 35.4% specificity.[vi] Thus, it is articulate that transvaginal ultrasound is not ideal for predicting disease, and so women with postmenopausal bleeding and thickened endometrium on imaging should undergo sampling.[seven]
All the same, endometrial cancer can nowadays with thin endometrium in rare cases, then recurrent or persistent postmenopausal bleeding should prompt pathologic evaluation. Additionally, these cutoffs practise not consider personal risk factors for endometrial cancer, such every bit obesity, diabetes, family unit history, or genetic conditions that may raise one's suspicion for endometrial cancer despite a thin endometrial lining.[5]
The gold standard for diagnosis or exclusion of endometrial cancer would exist endometrial sampling via dilation and curettage. In-function endometrial biopsy is besides an selection but is not equally accurate every bit it is often performed blindly. However, both procedures are invasive, and patients do not always well tolerate in-role endometrial biopsy. Ultrasonography of the pelvis, specifically transvaginal sonography, tin can be an of import screening tool to determine which patients crave sampling, either bullheaded or targeted.
Transvaginal ultrasound to evaluate the endometrium tin can have its limitations. For example, if a patient has a history of uterine fibroids, adenomyosis, obesity, or prior surgeries, it can be difficult to assess the endometrial lining fully. In those cases, endometrial sampling, function hysteroscopy, or saline-infusion sonogram should be performed as the side by side footstep in evaluation.
Incidental Thickened Endometrium
At times, postmenopausal women volition undergo pelvic ultrasound for indications other than postmenopausal bleeding and are found to have an endometrial thickness greater than 4mm. In these asymptomatic women, the guidelines are not as clear as to when sampling should be performed. Smith-Bindman et al. concluded that an endometrial thickness greater than 11mm places an asymptomatic postmenopausal woman at approximately vi.7% risk of cancer, similar to an approximate 7.3% risk of cancer in a woman with postmenopausal bleeding and an endometrium measuring 5mm.[8][nine][8]
Retrospective studies in asymptomatic women who underwent transvaginal ultrasound and endometrial sampling also support a cutoff of 11mm. Nevertheless, they have establish poorer sensitivity for detecting endometrial cancer than compared to symptomatic women.[10] Thus, while an 11mm cutoff for asymptomatic women may be a helpful starting betoken, physicians should still exercise clinical judgment when deciding how to manage an incidentally found thickened endometrium in a postmenopausal patient. In a nutshell, ACOG recommends that the decision to evaluate asymptomatic postmenopausal women with thickened endometrial stripe needs to be an individualized, case-by-case determination based on run a risk factors and patient characteristics rather than the endometrial thickness cut off used for postmenopausal bleeding patients.
Adnexal Masses
Adnexal masses are another frequent indication for a pelvic ultrasound in postmenopausal females. While most often constitute incidentally, adnexal masses may be felt on exam or crusade symptoms such equally pain, pressure, increasing abdominal girth, or fatigue.
Nigh adnexal masses are beneficial, but with increasing age, there is an increasing business organisation for ovarian carcinoma: it is estimated that women over 65 years old are 6 times more likely to receive a diagnosis of ovarian cancer than women nether 65 years onetime.[xi] Unfortunately, the presenting symptoms of ovarian carcinoma are vague, usually diagnosed at an avant-garde stage. Thus, there is great involvement in distinguishing beneficial from malignant adnexal masses. However, due to the relatively low prevalence of ovarian cancer, loftier prevalence of benign cysts, and poor specificity of ultrasound, routine yearly ultrasonography on all postmenopausal women equally a screening tool for ovarian cancer is not indicated.
The postmenopausal ovary is non necessarily totally quiescent. Simple cysts up to 1cm in diameter may exist seen in up to 1-fifth of postmenopausal women and practise non need to be followed. Too, simple cysts upwardly to 3cm in diameter can exist seen and often spontaneously regress.[3] Electric current guidelines suggest that elementary cysts between 1cm and 7cm in diameter should be followed at least yearly by ultrasound in a postmenopausal patient.[12] Larger or more complex cysts may crave evaluation through additional imaging or surgery.
Granberg et al. beginning showed that an ovarian mass's gross appearance could be related to its malignancy risk. Past evaluating over thousand tumors, they were able to find that unilocular cysts are associated with a 0.3% adventure of malignancy, complex multiloculated cysts are associated with a 36% chance of malignancy, and predominantly solid cysts are associated with a 39% chance of malignancy.[13] Particularly with high prototype quality in the transvaginal setting, pelvic ultrasound tin can thus be used to characterize the morphology of adnexal masses and potentially stratify the risk of malignancy.
However, descriptions of adnexal masses on ultrasound tin frequently be subjective. There have been several scoring systems proposed to standardize the sonographic evaluation of adnexal masses. These systems oft include overall morphology, ovarian volume, wall thickness, and the presence of internal nodules or papillary excrescences or septations. A review by Myers et al. institute that the virtually oft used scoring systems had a pooled sensitivity and specificity for detecting malignancy of 82-91% and 68-77%, respectively.[14]
The International Ovarian Tumor Analysis (IOTA) group first aimed to standardize sonographic descriptions of adnexal masses. In doing and so, they developed 2 related scoring systems that take been validated. The first, the IOTA Unproblematic Rules, involves two sets of ultrasound characteristics for benign and malignant masses (see tabular array 1).[15]
Table 1: IOTA Simple Rules
| Benign features | Cancerous features |
| Unilocular cyst | Irregular solid tumor |
| Solid component <7mm in diameter | Ascites |
| Presence of audio-visual shadows | ≥4 papillary structures |
| Smooth multilocular tumor with the largest bore <10cm | Irregular multilocular mass >10cm in bore |
| No detectable color Doppler signal | Potent color Doppler indicate |
If i or more malignant features are present with no benign features, then the mass is considered malignant. If 1 or more beneficial features are present with no malignant features, then the mass can exist considered benign. If either of those weather is not met, and so the issue is inconclusive.[16] In a meta-analysis, these simple rules were 93% sensitive and 81% specific for detecting malignancy.[17] More than recently, the IOTA group has developed the ADNEX (Cess of Dissimilar Neoplasias in the Adnexa) model to more than specifically predict whether an adnexal mass is beneficial, borderline, early on stage-malignant, late-phase malignant, or metastatic. This model uses similar ultrasound criteria as the Uncomplicated Rules and clinical criteria to calculate risk (run into table 2).[fifteen]
Tabular array 2: ADNEX Model Criteria
| Age |
| Serum CA-125 |
| Type of center (oncology referral centre or general hospital) |
| Maximum bore or lesion |
| The proportion of solid tissue (diameter of the largest solid component divided by maximum bore of the lesion) |
| Presence of > 10 cyst locules (yes/no) |
| No. of intracavitary papillary projections (0, 1, 2, 3, >iii) |
| Presence of acoustic shadows (yes/no) |
| Presence of ascites (yes/no) |
The ADNEX model was found to accept either comparable or superior sensitivity and specificity to both the Elementary Rules and other existing scoring systems in external validation studies. At malignancy take a chance cutoffs of 1%, 10%, and 30%, sensitivities approach 100%, 97% and 86%, respectively. Similarly, at malignancy risk cutoffs of i%, 10%, and 30%, near 12%, 75%, and 89%, respectively.[eighteen][nineteen][18] Thus, the IOTA Simple Rules and ADNEX model are promising methods for standardizing the sonographic evaluation of adnexal masses in postmenopausal patients. Notwithstanding, these algorithms are not yet widely implemented inside the United States, partly because they were validated in Europe. Proponents of the IOTA algorithms are hopeful that their use may become more widespread in the United States and better patients' triage and treatment with adnexal masses.
Nonstandard descriptions of adnexal masses notwithstanding, at that place can be other challenges with evaluating the postmenopausal adnexa by ultrasound. As mentioned before, postmenopausal ovaries are atrophic and may be difficult to visualize. Additionally, other structures within the pelvis, such as the bowel, fallopian tubes, and peritoneal cysts, may mimic the ovaries on imaging.
Contraindications
There are few contraindications to transvaginal ultrasound. Virginal patients or patients with meaning vaginal atrophy may not be able to tolerate a transvaginal ultrasound.
Equipment
An ultrasound auto forth with transabdominal and/or transvaginal ultrasound probes are needed.
Personnel
A sonographer or physician may perform an ultrasound. Oft it is a physician, either specializing in obstetrics and gynecology or radiology, who interprets the images.
Grooming
To better visualize the reproductive organs, a total float is preferred for a transabdominal scan compared to an empty bladder for a transvaginal scan.
Technique
While evaluating the female pelvis, it is customary to perform both transabdominal and transvaginal imaging.
Transabdominal
Transabdominal imaging of the postmenopausal pelvis is unremarkably of limited utility due to the normal atrophic changes of the reproductive organs. However, it may be helpful in patients that take an especially enlarged uterus or large pelvic masses due to a broader field of view compared to transvaginal imaging. It is also helpful in patients who are unable or unwilling to tolerate a transvaginal ultrasound. Transabdominal imaging is typically performed with a full float using 2.five to 5 MHz transducers.
Transvaginal
A transvaginal approach is the technique of choice when performing an ultrasound on a postmenopausal patient due to its loftier-resolution images. A transvaginal scan is performed afterwards emptying the float using a higher frequency transducer (5-viii MHz).
The endometrial thickness is calculated every bit the maximum anterior to the endometrium's posterior dimension when viewed in the sagittal or long-axis view of the uterus. Occasionally a small amount of anechoic fluid is noted in the endometrial culvert in postmenopausal women. In such cases, the endometrial thickness is measured by measuring the anterior and posterior dimensions of the endometrium separately and calculation them together.
These transabdominal and transvaginal imaging modalities are also useful in evaluating adnexal masses by identifying the origin, physical characteristics like size, contents like solid or cystic, septations, laterality, presence of papillary excrescences, presence or absenteeism of free fluid in the pelvis. Autonomously from identifying the gynecological causes for adnexal masses, ultrasound can also be used to identify other non-gynecological causes for adnexal masses like appendicitis or appendicular abscess or pelvic kidney or peritoneal inclusion cysts.
Saline Infusion Sonography
Saline-infusion sonography, or Sister, can be a helpful adjunct to transvaginal ultrasound in evaluating the lesions in the endometrial cavity or in patients with difficulty visualizing the endometrial lining. In Sis, a pocket-sized catheter is inserted through the neck into the endometrial culvert. Saline is so infused through the catheter while a transvaginal ultrasound is performed. The saline distends the endometrial cavity, allowing for intracavitary lesions such every bit endometrial polyps or submucosal fibroids to be seen more hands. In the case of postmenopausal bleeding, SIS tin help determine if the endometrium is but focally thickened (in the case of a polyp) or more globally thickened (in the case of hyperplasia or malignancy).
Doppler
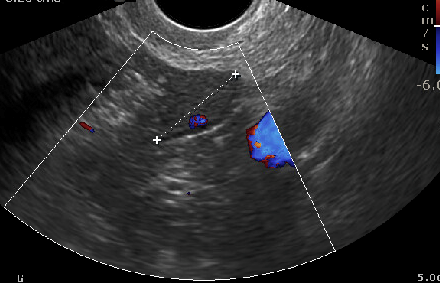
Doppler ultrasound, with which blood flow through vessels can be evaluated, is another technique that may exist applied when imaging a postmenopausal patient. Doppler can be helpful in the differentiation of a benign from a malignant adnexal mass.[twenty]
Clinical Significance
As noted higher up, pelvic ultrasound in the postmenopausal patient is key in evaluating postmenopausal bleeding and adnexal masses. Thus, these imaging studies tin can be cardinal in the early detection of endometrial or ovarian cysts and atomic number 82 to improved outcomes in patients.
Enhancing Healthcare Team Outcomes
Pelvic ultrasound in the postmenopausal female is the imaging study of choice to evaluate the uterus, endometrium, cervix, and adnexa. It is important to recognize when a pelvic ultrasound should be performed in a postmenopausal patient. Knowing the indications for a pelvic ultrasound and how to interpret the imaging findings can ameliorate patient outcomes. For the sonographer performing the ultrasound, it is important to have the proper technique to evaluate possible pathologies fully.
If a standard transvaginal ultrasound is inconclusive or suboptimal, knowing how to perform saline-infused sonohysterography may add diagnostic information. The ordering or interpreting doc needs to know when an ultrasound is indicated for a postmenopausal patient to decrease the demand for potentially unnecessary further testing. Additionally, it is important to know how to interpret the ultrasound findings in a postmenopausal patient. In particular, knowing the appropriate endometrial thickness in a postmenopausal patient with haemorrhage and apropos ultrasound features of an adnexal mass can assistance determine the need for further workup and guide management in these patients.
With open communication and collaboration betwixt the healthcare team members (including clinicians, mid-level practitioners, specialists, and nurses), a properly performed and interpreted pelvic ultrasound in the postmenopausal adult female can take important diagnostic consequences and lead to improved outcomes in patients.
(Click Image to Enlarge)
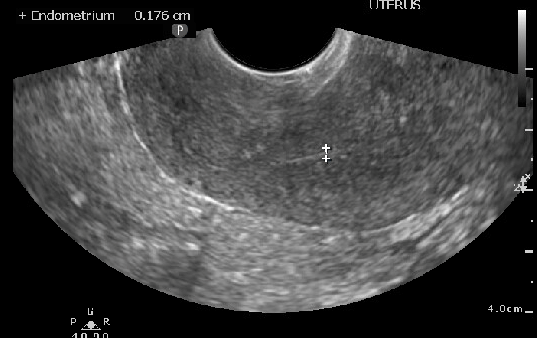
Ultrasound image measuring thin endometrial stripe in postmenopausal patient
Contributed past Laveena Kondagari, MD
(Click Image to Overstate)
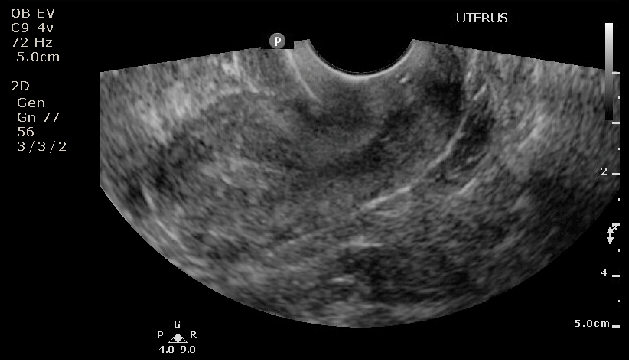
Ultrasound of Postmenopausal patient with thickened endometrial stripe
Contributed by Laveena Kondagari, MD
(Click Image to Overstate)
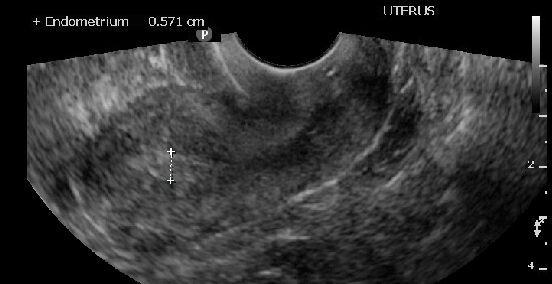
Measuring endometrial stripe in sagittal view of the uterus
Contributed past Laveena Kondagari ,Medico
(Click Image to Enlarge)
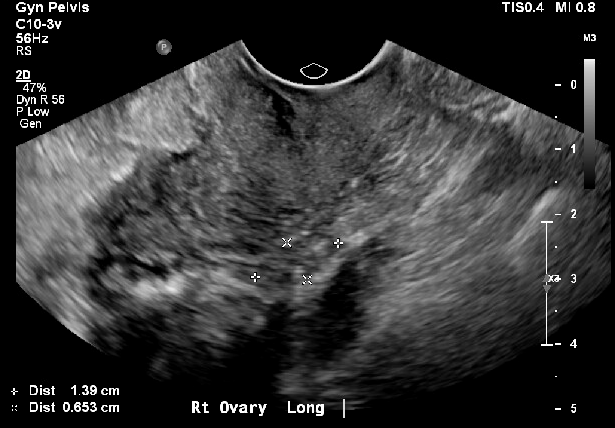
Postmenopausal patient with small follicles
Contributed by Laveena Kondagari, MD
(Click Paradigm to Overstate)
Postmenopausal ovary with no follicles
Contributed by Laveena Kondagari, MD
References
[2]
Merz E,Miric-Tesanic D,Bahlmann F,Weber G,Wellek S, Sonographic size of uterus and ovaries in pre- and postmenopausal women. Ultrasound in obstetrics [PubMed PMID: 8932630]
[iii]
Langer JE,Oliver ER,Lev-Toaff Equally,Coleman BG, Imaging of the female person pelvis through the life wheel. Radiographics : a review publication of the Radiological Society of Northward America, Inc. 2012 Oct; [PubMed PMID: 23065159]
[4]
Goldstein RB,Bree RL,Benson CB,Benacerraf BR,Bloss JD,Carlos R,Fleischer AC,Goldstein SR,Hunt RB,Kurman RJ,Kurtz AB,Laing FC,Parsons AK,Smith-Bindman R,Walker J, Evaluation of the woman with postmenopausal bleeding: Club of Radiologists in Ultrasound-Sponsored Consensus Conference statement. Journal of ultrasound in medicine : official journal of the American Constitute of Ultrasound in Medicine. 2001 Oct; [PubMed PMID: 11587008]
[5]
ACOG Commission Opinion No. 734: The Function of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Haemorrhage. Obstetrics and gynecology. 2018 May; [PubMed PMID: 29683909]
[6]
Timmermans A,Opmeer BC,Khan KS,Bachmann LM,Epstein E,Clark TJ,Gupta JK,Bakour SH,van den Bosch T,van Doorn HC,Cameron ST,Giusa MG,Dessole S,Dijkhuizen FPHLJ,Ter Riet G,Mol BWJ, Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-assay. Obstetrics and gynecology. 2010 Jul; [PubMed PMID: 20567183]
[vii]
Smith-Bindman R,Kerlikowske K,Feldstein VA,Subak Fifty,Scheidler J,Segal Grand,Make R,Grady D, Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998 November four; [PubMed PMID: 9809732]
[8]
Smith-Bindman R,Weiss E,Feldstein 5, How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal haemorrhage. Ultrasound in obstetrics [PubMed PMID: 15386607]
[nine]
Menzies R,Wallace S,Ennis Thou,Bennett A,Jacobson Yard,Yip G,Wolfman West, Significance of abnormal sonographic findings in postmenopausal women with and without haemorrhage. Periodical of obstetrics and gynaecology Canada : JOGC = Periodical d'obstetrique et gynecologie du Canada : JOGC. 2011 Sep; [PubMed PMID: 21923992]
[ten]
Seckin B,Cicek MN,Dikmen AU,Bostancı EI,Muftuoglu KH, Diagnostic value of sonography for detecting endometrial pathologies in postmenopausal women with and without bleeding. Journal of clinical ultrasound : JCU. 2016 Jul eight; [PubMed PMID: 26857098]
[11]
Rauh-Hain JA,Melamed A,Buskwofie A,Schorge JO, Adnexal mass in the postmenopausal patient. Clinical obstetrics and gynecology. 2015 Mar; [PubMed PMID: 25565080]
[12]
Levine D,Brown DL,Andreotti RF,Benacerraf B,Benson CB,Brewster WR,Coleman B,Depriest P,Doubilet PM,Goldstein SR,Hamper UM,Hecht JL,Horrow M,Hur HC,Marnach Yard,Patel Physician,Platt LD,Puscheck Due east,Smith-Bindman R, Direction of asymptomatic ovarian and other adnexal cysts imaged at The states: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010 Sep; [PubMed PMID: 20505067]
[thirteen]
Granberg S,Wikland M,Jansson I, Macroscopic characterization of ovarian tumors and the relation to the histological diagnosis: criteria to be used for ultrasound evaluation. Gynecologic oncology. 1989 Nov; [PubMed PMID: 2680797]
[14]
Myers ER,Bastian LA,Havrilesky LJ,Kulasingam SL,Terplan MS,Cline KE,Gray RN,McCrory DC, Direction of adnexal mass. Evidence report/technology assessment. 2006 Feb; [PubMed PMID: 17854238]
[15]
Abramowicz JS,Timmerman D, Ovarian mass-differentiating beneficial from malignant: the value of the International Ovarian Tumor Analysis ultrasound rules. American journal of obstetrics and gynecology. 2017 December; [PubMed PMID: 28735703]
[xvi]
Timmerman D,Ameye L,Fischerova D,Epstein Eastward,Melis GB,Guerriero Southward,Van Holsbeke C,Savelli L,Fruscio R,Lissoni AA,Testa AC,Veldman J,Vergote I,Van Huffel South,Bourne T,Valentin 50, Uncomplicated ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA grouping. BMJ (Clinical research ed.). 2010 Dec 14; [PubMed PMID: 21156740]
[17]
Kaijser J,Sayasneh A,Van Hoorde K,Ghaem-Maghami Southward,Bourne T,Timmerman D,Van Calster B, Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: a systematic review and meta-analysis. Homo reproduction update. 2014 May-Jun; [PubMed PMID: 24327552]
[18]
Viora E,Piovano E,Baima Poma C,Cotrino I,Castiglione A,Cavallero C,Sciarrone A,Bastonero South,Iskra L,Zola P, The ADNEX model to triage adnexal masses: An external validation written report and comparison with the IOTA two-stride strategy and subjective assessment by an experienced ultrasound operator. European journal of obstetrics, gynecology, and reproductive biological science. 2020 Apr; [PubMed PMID: 32146226]
[19]
Nohuz E,De Simone Fifty,Chêne K, Reliability of IOTA score and ADNEX model in the screening of ovarian malignancy in postmenopausal women. Journal of gynecology obstetrics and human reproduction. 2019 Feb; [PubMed PMID: 29709594]
[xx]
Kupesic South,Kurjak A,Hajder E, Ultrasonic assessment of the postmenopausal uterus. Maturitas. 2002 Apr 25; [PubMed PMID: 12034512]
How Does The Size Of The Uterus Change From Prepuberty To Menopause?,
Source: https://www.statpearls.com/ArticleLibrary/viewarticle/128652
Posted by: davisfacheneve.blogspot.com


0 Response to "How Does The Size Of The Uterus Change From Prepuberty To Menopause?"
Post a Comment